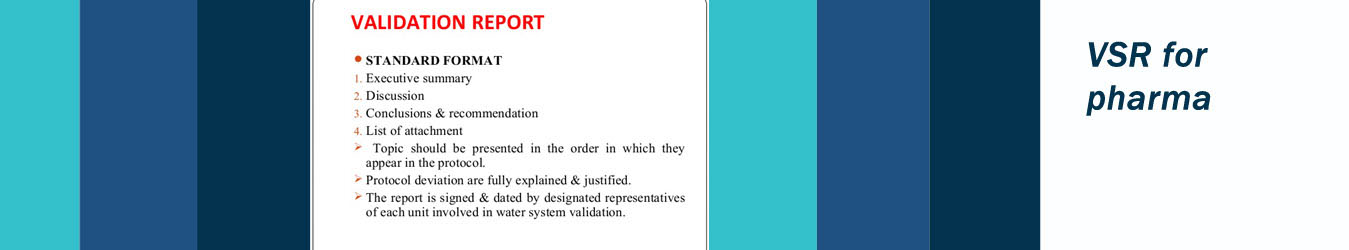
A VSR means summary of all planned activities, their success or failure, and any deviations from the expected results or plans encountered. A satisfactory resolution should be provided to explain and resolve any deviations encountered.
The validation summary report(VSR) brings together all of the documentation collected throughout the whole of the life cycle and presents a recommendation for management approval when the system is validated. The document should also contain a formal release statement to allow the system to be used for regulated work.




